Nucleotides
- Details
- Last Updated: Friday, 14 July 2023 09:27
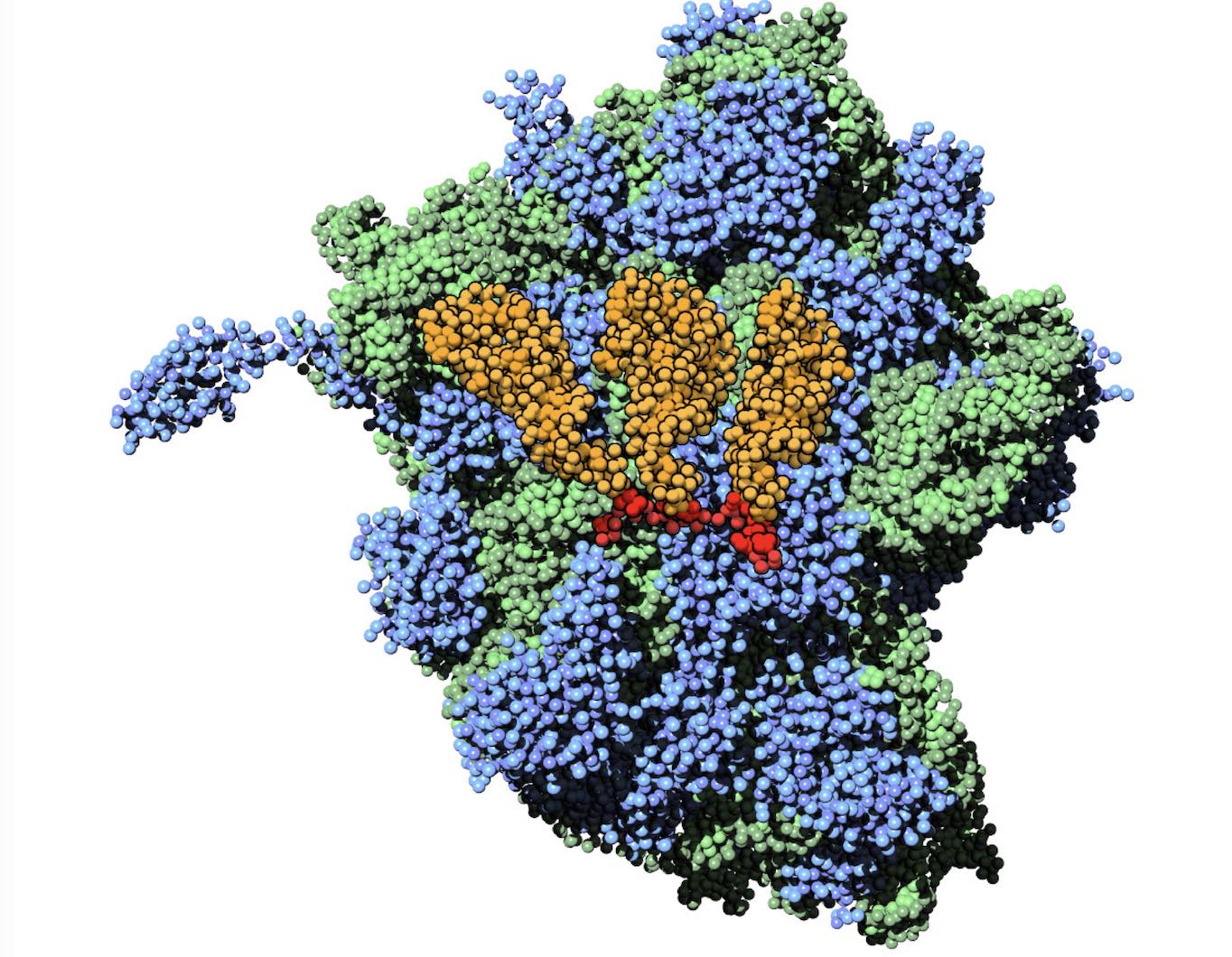 Polynucleotides (DNA, RNA) form a fundamental class of biomolecules. Given their importance, Martini models for both DNA [1] and RNA [2, see Figure depicting a Martini ribosome] have been developed. Parameters for nucleotide cofactors are also available [3,4], as well as a dedicated tool to analyze nucleoside packing [5]. Given the limitations of Martini with respect to modeling the directionality of hydrogen bonds, nucleotide folding and hybridization events can not be captured. As for the protein model, an elastic network is required to keep the overall structure in place.
Polynucleotides (DNA, RNA) form a fundamental class of biomolecules. Given their importance, Martini models for both DNA [1] and RNA [2, see Figure depicting a Martini ribosome] have been developed. Parameters for nucleotide cofactors are also available [3,4], as well as a dedicated tool to analyze nucleoside packing [5]. Given the limitations of Martini with respect to modeling the directionality of hydrogen bonds, nucleotide folding and hybridization events can not be captured. As for the protein model, an elastic network is required to keep the overall structure in place.
Despite this limitation, Martini can be used to study the interaction of nucleotides with other biomolecules, for instance, a DNA origami nanopore in a lipid membrane [6], DNA scaffolded nanodiscs [7], DNA-lipid formulations (lipoplexes) for gene delivery [8,9], or complexation of polymers with RNA [10] and DNA [11]. Recently, whole-cell genome has been modeled with Martini [13].
The Martini nucleotide force field has also been integrated into the HADDOCK framework to predict protein-DNA interfaces [12].
[1] J.J. Uusitalo, H.I. Ingólfsson, P. Akhshi, D.P. Tieleman, S.J. Marrink.Martini coarse-grained force field: extension to DNA. JCTC 11:3932-3945, 2015. open access
[3] F.M. Sousa, L.M.P. Lima, C. Arnarez, M.M. Pereira, M.N. Melo. Coarse-Grained Parameterization of Nucleotide Cofactors and Metabolites: Protonation Constants, Partition Coefficients, and Model Topologies. J. Chem. Inform. Modeling 61:335-346, 2021. DOI: 10.1021/acs.jcim.0c01077t
[4] C.F.E. Schroer, L. Baldauf, L. van Buren, T.A. Wassenaar, M.N. Melo, G. Koenderink, S.J. Marrink. Charge-dependent interactions of monomeric and filamentous actin with lipid bilayers. PNAS,
[5] I. Faustino, S.J. Marrink. cgHeliParm: analysis of dsDNA helical parameters for coarse-grained MARTINI molecular dynamics simulations. Bioinformatics, 33:3813-3815, 2017. abstract
[6] V. Maingi, J.R. Burns, J.J. Uusitalo, S. Howorka, S.J. Marrink, M.S.P. Sansom. Stability and dynamics of membrane-spanning DNA nanopores. Nature Comm. 8:14784, 2017. open access
[7] V. Maingi, P.W.K. Rothemund. Properties of DNA- and Protein-Scaffolded Lipid Nanodiscs. ACS Nano 15:751–764, 2021. https://doi.org/10.1021/acsnano.0c07128
[8] B.M.H. Bruininks, P.C.T. Souza, S.J. Marrink. A Practical View of the Martini Force Field. Biomolecular Simulations, 105-127, 2019. doi:10.1007/978-1-4939-9608-7_5 , pdf-reprint.
[9] B.M.H. Bruininks, P.C.T. Souza, H. Ingolfsson, S.J. Marrink. A molecular view on the escape of lipoplexed DNA from the endosome. eLife, 9:e52012, 2020. doi.org/10.7554/eLife.52012
[10] F. Stojceski, G. Grasso, L. Pallante, A. Danani. Molecular and Coarse-Grained Modeling to Characterize and Optimize Dendrimer-Based Nanocarriers for Short Interfering RNA Delivery. ACS Omega 5:2978-2986, 2020.
DOI: 10.1021/acsomega.9b03908
[11] S. Mahajan, T. Tang. Polyethylenimine–DNA Ratio Strongly Affects Their Nanoparticle Formation: A Large-Scale Coarse-Grained Molecular Dynamics Study. JPC-B 123:9629-9640, 2019
DOI: 10.1021/acs.jpcb.9b07031
[12] R.V. Honorato, J. Roel-Touris, A.M.J.J. Bonvin. MARTINI-Based Protein-DNA Coarse-Grained HADDOCKing. Front. Mol. Biosci., 2019. https://doi.org/10.3389/fmolb.2019.00102
[13] B.R. Gilbert, Z.R. Thornburg, T.A. Brier, J.A. Stevens, F. Grunewald, J.E. Stone, S.J. Marrink, Z.A. Luthey-Schulten. Dynamics of Chromosome Organization in a Minimal Bacterial Cell. Front. Cell Dev. Biol., 2023, online. doi:10.3389/fcell.2023.1214962


























































































































































